Molarity Practice Problems with Answers and Tutorial
- Posted by Brian Stocker MA
- Date April 7, 2014
- Comments 20 comments
Molarity
Molarity is the measure of the concentration of a substance in a solution, given in terms of the amount of substance per unit volume of the solution. Molarity questions are on the HESI and the NLN PAX
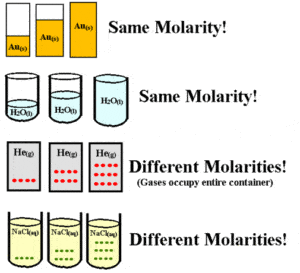
How to Solve Molarity Practice Problems
Molarity is also called, amount-of-substance concentration, amount concentration, substance concentration, or simply concentration.
The Molarity of a solution simply means the amount of moles contained in every liter of a solution. To better understand the concept of molarity of a solution it is necessary to first understand some related terms.
* A solute – is the substance that is being dissolved such as sugar or mercury.
* A solvent – refers to the liquid that the solute is being dissolved in.
* A solution – refers to the mixture of the solvent and the solute so that solution equals solvent plus solute.
The Molarity of the solution is thus a measurement of the molar concentration of the solute in the solution. The molarity of a solution is measured in moles of solute per liter of solution, or mol/liter. For example, if the molarity of a mercury solution is 1M, it simply means that there is 1 mole of sugar contained in every 1 liter of the solution.
The formula for molarity is = moles of solute/total liters of solution
Here is a typical molarity practice problem:
If 2 moles of salt is dissolved to form 1 liter of solution, calculate the molarity of the solution.
a. 1 M solution
b. 1.5 M solution
c. 2 M solution
d. 2.5 M solution
The formula for calculating molarity when the moles of the solute and liters of the solution are given is = moles of solute/ liters of solution.
Moles of Solute = 2 moles of sugar
Solution liters = 1 liters
The molarity of the solution = 2 moles of solvent/1 liters of solution = 2 M solution.
Molarity Practice Problems
1. Calculate the molarity of a sugar solution if 4 liters of the solution contains 8 moles of sugar?
a. 0.5 M
b. 8 M
c. 2 M
d. 80 M
2. What is the molarity of a solution containing 5 moles of solute in 250 milliliters of solution?
a. 20 M
b. 15 M
c. 0.104 M
d. 1.25 M
3. How many moles of NAOH are needed to dissolve in water to make 4 liters of a 2.0 M solution?
a. 0.50 M
b. 2 M
c. 8 mol
d. 0.5 mol
4. How many moles of Na are needed to make 4.5 liters of a 1.5 M Na solution?
a. 6.75 mol
b. 0.33 M
c. 0.33 mol
d. 3 M
5. Molarity of a solution can be defined as the:
a. atomic mass of an element
b. moles of solution per liter of solute
c. moles of solute per liter of solution
d. mass of solvent per liter of solution
6. To calculate the Molarity of a solution when the solute is given in grams and the volume of the solution is given in milliliters, you must first:
a. Convert grams to moles, but leave the volume of solution in milliliters
b. Convert volume of solution in milliliters to liters, but leave grams to moles
c. Convert grams to moles, and convert volume of solution in milliliters to liters
d. None of the above
7. The molarity of an aqueous solution of CaCl is defined as the
a. moles of CaCl per milliliter of solution
b. grams of CaCl per liter of water
c. grams of CaCl per milliliter of solution
d. moles of CaCl per liter of solution
Answer Key
1. C
Molarity = moles of solute/liters of solution = 8/4 = 2
2. A
First convert 250 ml to liters, 250/1000 = 0.25 then calculate molarity = 5 moles/ 0.25 liters = 20 M
3. C
A solution with molarity 2 requires 2 M of NAOH per liter.
So, 4 X 2 = 8 M
4. A
A solution of molarity 1.5 M, requires 1.5 mol of Na to every litre of solvent.
1.5 mol of Na into 1L renders 1L of 1.5M solution
Therefore, multiply the molarity of the desired solution by the end volume required:
4.5L requires 6.75 mol of Na, as 1.5(M)*4.5(L)=6.75 (mol).
5. C
6. C
7. D
Date Published: Monday, April 7th, 2014
Date Modified: Wednesday, July 12th, 2023
You may also like

Listening Comprehension Practice – Solving a Problem


20 Comments
It was very easy . I want some difficult questions ..
Acuerdo mucho con tu opinion.
It was good, even though they were easy, it’s great to have problems
It is very easy
in 3 rd question it will be NaOH but it is NAOH
thanks!
yes.you are correct
thanks HAPPY NEW YEAR
It’s very eazy dont know what I waz stressing about
it was easy . Please upload some difficult and tricky questions.
All questions r easy …I want more questions
thanks.now i know basic molarity issues.do you mind bringing a little bit complex ones?
Easy questions and these type of questions increases the confidence level of the student which is quite good . Questions are depends on the basic knowledge of the topic molarity throughout.
This was great! Thank you!
it was easy and good, thank u .
it was quite easy though
Thanks
thank you sir
It’s too easy to solve but I miss answered q no 6
Thank you for Jon My son